
ピックアップとドロップオフが含まれています


Hyperbaric Oxygen Therapy (HBOT) involves breathing pure oxygen in a pressurized chamber, typically at pressures higher than normal atmospheric levels. This treatment is utilized to address various medical conditions and offers numerous potential benefits.
During an HBOT session, patients inhale oxygen at levels greater than those found at sea level, which helps facilitate tissue healing and repair.
HBOT is commonly used to treat conditions such as decompression sickness in divers, non-healing wounds, carbon monoxide poisoning, and radiation injuries. Oxygen therapy has shown to be effective in treating chronic headaches as well.
Originating in the USA, Hyperbaric Oxygen Therapy is used to stimulate blood circulation and oxygenation within the body for healing and recovery. Backed by evidence-based research, it has been proven that the brain is highly sensitive to changes in circulating oxygen levels, with low or absent oxygen quickly leading to unconsciousness or permanent brain damage.

As of July 2021, the FDA has cleared hyperbaric chambers for the following disorders:
Decompression sickness, also known as “the bends,” occurs when nitrogen bubbles form in the bloodstream due to rapid decompression, such as after diving. HBOT is the primary treatment for this condition. By increasing atmospheric pressure and oxygen concentration, HBOT shrinks the nitrogen bubbles and rapidly restores oxygen to affected tissues, preventing or reversing neurological and musculoskeletal damage.
One of the most established uses of HBOT is in the treatment of diabetic foot ulcers and other chronic non-healing wounds. In these patients, poor circulation results in hypoxic (low-oxygen) tissues, slowing down wound healing. HBOT dramatically enhances oxygen delivery to these tissues, stimulating fibroblast proliferation, collagen synthesis, and new blood vessel formation (angiogenesis), all critical to wound repair.
Carbon monoxide binds to hemoglobin more readily than oxygen, leading to tissue hypoxia and potential brain damage. HBOT works by rapidly removing CO from the bloodstream, restoring normal oxygen transport and reducing the risk of permanent neurological damage. It’s an emergency treatment that can be lifesaving.
Healthy tissues surrounding a tumor can be affected by radiation therapy, sometimes leading to delayed complications known as radiation injuries. Hyperbaric Oxygen Therapy (HBOT) is FDA-approved for treating these latent radiation-induced tissue damages, as it helps repair and regenerate the affected areas.
Severe anemia when blood transfusions cannot be used.
Severe and large burns treated at a specialized burn center.
Complete hearing loss that occurs suddenly and without any known cause.
When sudden and painless in one eye due to blockage of blood flow.
HBOT is being studied for other conditions, including COVID-19. However, at this time, the FDA has not cleared or authorized the use of any HBOT device to treat COVID-19 or any conditions beyond those listed above. The website, clinicaltrials.gov, has more information on HBOT clinical trials for COVID-19 and other conditions.
Though not yet an FDA-approved indication, HBOT has been reported to alleviate chronic headaches, possibly due to its cerebrovascular effects, improving oxygen supply to the brain and reducing neuroinflammation and pain perception.
HBOT is being actively investigated for treating TBI, particularly mild TBI or post-concussion syndrome. It enhances oxygenation in damaged brain tissues, supports mitochondrial recovery, and promotes neurogenesis and angiogenesis. These processes may help reduce cognitive symptoms such as memory loss, fatigue, and brain fog.
In patients recovering from a stroke, HBOT can restore oxygen to areas with reduced blood flow and aid in neurological recovery. Studies show improvements in memory, cognitive function, and quality of life, likely due to improved brain perfusion and reduced inflammation.
Avascular necrosis (AVN), particularly of the femoral head, is a degenerative condition caused by reduced blood supply to bone tissue, leading to cell death and eventual joint collapse. Risk factors include corticosteroid use, alcohol abuse, diabetes, and decompression sickness.
HBOT is being studied as a non-surgical intervention for AVN by enhancing oxygen delivery to ischemic bone tissue, reducing edema, and promoting angiogenesis and bone remodeling. In the referenced 30-year retrospective study from Fremantle and Fiona Stanley Hospitals, patients who received ≥20 HBOT sessions showed improvements in MRI findings, symptom relief, and potentially reduced surgical needs.
While promising, HBOT for AVN remains an off-label and investigational use, requiring more high-quality randomized controlled trials before it can be considered an established therapy.
HBOT has been shown to help individuals experiencing prolonged post-COVID symptoms, including fatigue, cognitive dysfunction (“brain fog”), and breathlessness. It reduces systemic inflammation and improves oxygenation, especially in underperfused areas of the brain and lungs.
HBOT may help slow or reverse biological aging by enhancing cellular and molecular repair processes. Notably, it can lengthen telomeres by up to 38% and reduce senescent (aged) cell count by up to 37%. It stimulates the proliferation and mobilization of stem cells, enhances antioxidant defense mechanisms, and encourages new capillary growth—all of which are associated with healthier aging and tissue regeneration.
HBOT significantly improves signs of skin aging. It reduces elastic fiber fragmentation, increases collagen density, promotes angiogenesis (new blood vessels), and reduces the number of senescent skin cells. These changes result in healthier, more youthful-looking skin and may delay the visible effects of aging.
HBOT promotes deeper, more restorative sleep by increasing oxygen availability in the brain. This enhances melatonin production, reduces sleep latency, and improves overall sleep architecture. Patients report better recovery and reduced fatigue. The therapy may benefit individuals with chronic insomnia or sleep-disordered breathing.
By increasing oxygen at the cellular level, HBOT enhances mitochondrial function and energy production, which can reduce symptoms of chronic fatigue. This is especially useful for individuals recovering from viral illnesses, including post-viral fatigue syndromes like Long COVID.
HBOT creates an oxygen-rich environment that is favorable for reproductive health by promoting angiogenesis and regulating oxidative stress. It can improve uterine lining quality and oxygen delivery to reproductive organs, thus enhancing the success rate of assisted reproductive technologies like IVF.
Hyperbaric oxygen therapy (HBOT) enhances tissue oxygenation by delivering 100% oxygen at elevated pressures and has shown promise in treating bone diseases. Research indicates that HBOT promotes bone healing by stimulating osteoblast activity, encouraging angiogenesis, and modulating inflammation and oxidative stress. It has demonstrated therapeutic benefits in fracture healing, bone defect repair, and osteoporosis by targeting key bone signaling pathways. While clinical studies support its efficacy, the optimal HBOT protocols for different bone conditions are still being determined.
HBOT can accelerate healing from muscle strains, ligament tears, and bone injuries. It reduces inflammation, promotes tissue remodeling, and supports cell regeneration. Elite athletes have used HBOT to recover from fatigue and injury more quickly during competitions.
HBOT has shown promising effects in some children with autism, particularly in improving cerebral perfusion and reducing neuroinflammation. At low pressures (up to 1.5 ATA), it is well-tolerated and has led to behavioral and language improvements in some clinical studies, though more research is needed.
HBOT reduces pro-inflammatory cytokines like TNF-α and IL-6 while boosting anti-inflammatory cytokines like IL-10. This makes it beneficial for chronic inflammatory conditions such as eczema, arthritis, or other systemic inflammatory states. It also improves vascular stability and immune regulation.
In cosmetic procedures, especially dermal fillers or minimally invasive treatments, HBOT has been used to prevent or treat skin necrosis and tissue ischemia. It accelerates healing, improves blood flow to compromised areas, and reduces the risk of scarring and complications.
HBOT supports the immune system in fighting off difficult-to-treat infections by enhancing white blood cell function, increasing oxygen levels in infected tissues, and even suppressing the growth of certain anaerobic bacteria. This includes infections such as necrotizing fasciitis and chronic osteomyelitis.




The most measured man in the world, Bryan Johnson, reveals his results from 90 days of hyperbaric oxygen therapy.
Bryan Johnson, a venture capitalist famous for investing millions into age-reversal technologies, recently shared the results of undergoing 60 sessions of hyperbaric oxygen therapy (HBOT). Best known for its use in treating decompression sickness in divers, HBOT is also FDA-approved for various medical conditions like vision loss, hearing loss, and wound healing. However, its potential as an anti-aging therapy remains under investigation.
HBOT involves breathing pure oxygen in a pressurized chamber, increasing oxygen levels in blood and tissue. Johnson, who frequently monitors his biological age markers, used his rigorous data tracking to evaluate the impact of HBOT on aging-related metrics—and the results, according to him, were impressive.
Over a 90-day period, Johnson completed 60 HBOT sessions using a hardshell chamber pressurized to 2 atmospheres. Each session lasted 90 minutes, during which he alternated between breathing 100% oxygen for 20 minutes and taking 5-minute breaks. He reported these sessions delivered significant improvements across multiple health indicators.
“HBOT ranks as one of the highest value health therapies I’ve done,” Johnson shared on X.
Johnson reported that HBOT completely eliminated systemic inflammation in his body. His high-sensitivity C-reactive protein (hsCRP) levels fell below detectable limits. In addition, his C-reactive protein metabolite (CRPm) levels ranked in the lowest 1% of individuals tested—suggesting a powerful anti-inflammatory effect.
HBOT is known to improve blood vessel formation (angiogenesis). Johnson’s vascular endothelial growth factor (VEGF) levels rose by 300%, supporting increased vascularization. Additionally, skeletal muscle oxygen saturation (SmO₂) more than doubled during exercise at 210 watts after 40 sessions.
Telomeres—protective caps at the end of chromosomes—shorten with age. Johnson found that HBOT boosted his telomerase activity to 7.7%, a level comparable to that of a 12-year-old. While his telomere length data was compromised by a lab error, he plans to retest and share those results later.
HBOT led to a 250% increase in short-chain fatty acids (SCFAs) and a 290% rise in n-butyrate levels—both essential for gut and metabolic health. Notably, the presence of Akkermansia muciniphila, a beneficial gut bacterium linked to strong intestinal barriers and metabolic balance, rose by 1000%.
In Alzheimer’s disease, elevated tau protein levels in the brain are associated with inflammation and cognitive decline. Johnson’s phosphorylated tau (TAU127) dropped by 28.6%, from 0.14 pg/mL to 0.1 pg/mL, a level well below the Alzheimer’s risk threshold (0.18 pg/mL). This suggests a reversal of brain aging biomarkers.
Johnson reported a reduction in his skin’s biological age from 39 to 38, based on skin texture, pore size, pigmentation, and other metrics. He also cited studies showing HBOT can increase collagen, blood flow, and reduce skin-cell senescence.
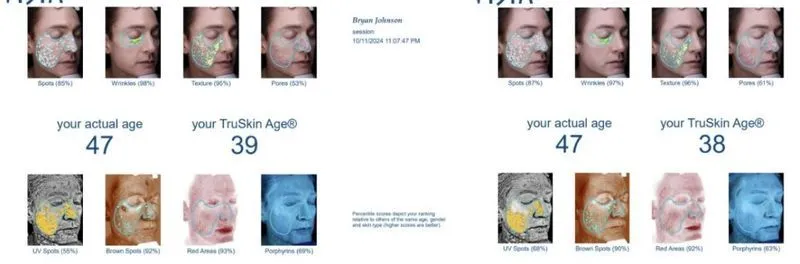
“The outcomes match what we observed in the scientific literature and what we predicted in deciding to do this therapy. What’s notable is that after achieving elite level biomarkers over the past four years, my team and I have struggled to find new therapies that meaningfully improve my biomarkers. HBOT achieved that,” said Johnson.
Johnson emphasized that the outcomes of his self-experiment align closely with existing scientific literature on HBOT. Given that he had already optimized many of his health markers over the past four years, he was surprised to find HBOT delivering additional meaningful improvements.
“After achieving elite-level biomarkers... HBOT achieved that,” he wrote.
For those unable to afford HBOT, Johnson and researchers suggest an alternative: conscious breathing techniques that activate the parasympathetic nervous system. This system regulates inflammation and oxidative stress—key drivers of aging.
A study by Gerbarg and Brown (2016) recommends breathing at a rate of 4.5–6 breaths per minute to balance the autonomic nervous system and promote calm alertness. Breathing even slower (3 or fewer breaths per minute) can induce a meditative, deeply restorative state.

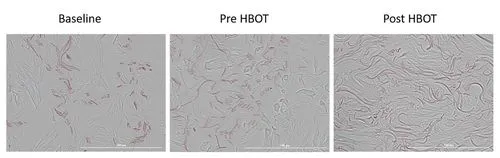
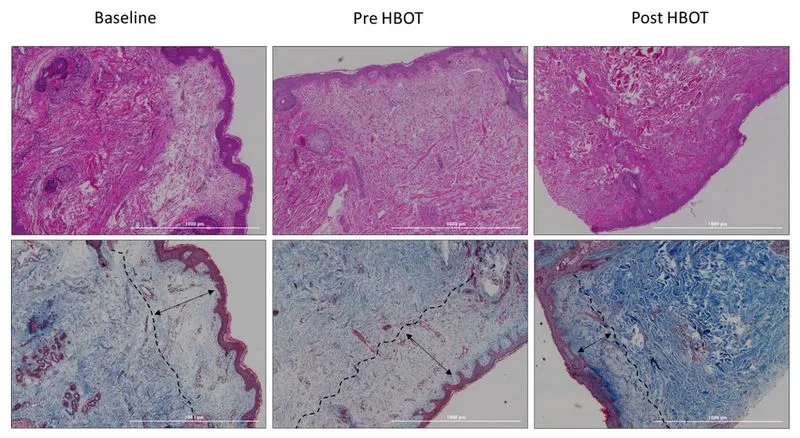
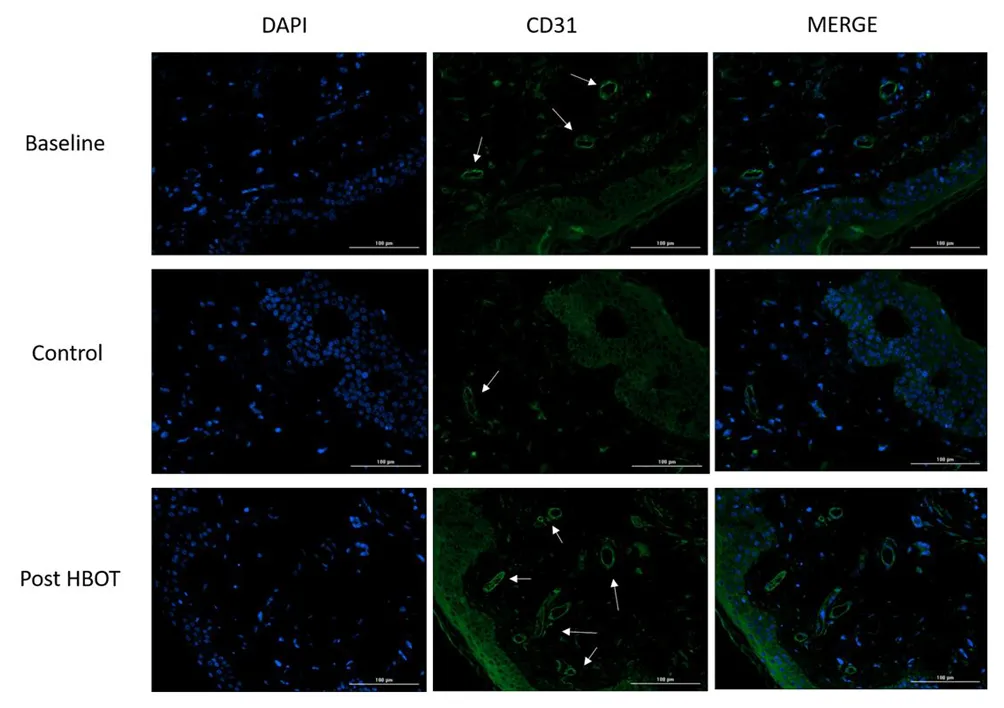
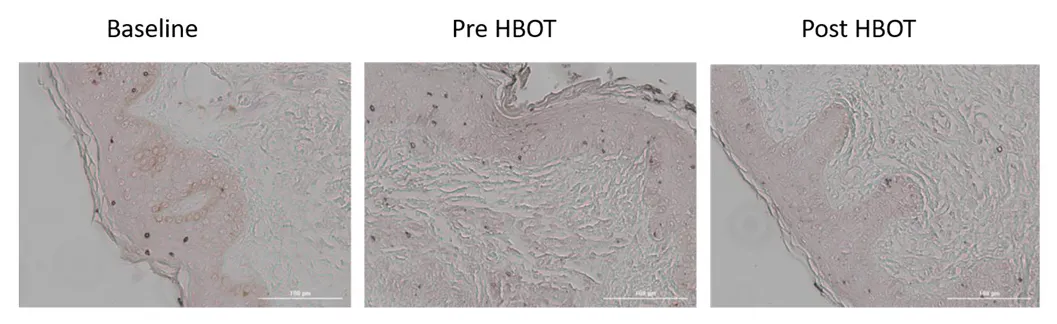
This study presents a research study evaluating the effects of hyperbaric oxygen therapy on skin aging in a normal aging population through skin biopsies and various physiological assessments.
The study revealed significant histological changes in the skin of participants after HBOT, indicating its potential to reverse some aging effects. 14
The study suggests that HBOT may exert its effects through mechanisms such as angiogenesis and clearance of senescent cells, which are critical in the aging process. 18
Therefore, in this study, Hyperbaric oxygen therapy (HBOT) has been shown to significantly improve skin aging in a healthy aging population by enhancing collagen density, elastic fiber length, and reducing aged, senescent skin cells.

This paper reports on a randomized controlled trial evaluating the effects of hyperbaric oxygen therapy on physical performance and cardiac perfusion in sedentary older adults.
The study investigates the impact of hyperbaric oxygen therapy (HBOT) on physical performance and cardiac function in sedentary older adults. Results indicate significant improvements in oxygen consumption and cardiac perfusion following HBOT.
A randomized controlled trial conducted from 2016 to 2020.
Involved 63 adults aged 64 and older, divided into HBOT (n=30) and control (n=33) groups.
Participants underwent 60 sessions of HBOT over 12 weeks, breathing 100% oxygen at 2 ATA for 90 minutes.
Primary endpoints included VO2Max and VO2Max/kg; secondary endpoints included cardiac perfusion and pulmonary function.
Significant increase in VO2Max/kg by 1.91 ± 3.29 ml/kg/min (p = 0.0034) with a net effect size of 0.455.
VO2 consumption at the first ventilatory threshold (VO2VT1) increased by 160.03 ± 155.35 ml/min (p < 0.001) with a net effect size of 0.617.
Global myocardial blood flow (MBF) increased from 0.34 ± 0.10 to 0.42 ± 0.19 ml/100 g/min (p = 0.008), with a large effect size of 0.797.
Global myocardial blood volume (MBV) increased from 0.53 ± 0.14 to 0.61 ± 0.22 ml/100 g (p = 0.009), with an effect size of 0.896.
Moderate correlations observed between MBV changes and VO2Max (r = 0.45, p = 0.043).
The study demonstrates that Hyperbaric Oxygen Therapy (HBOT) significantly improves physical performance metrics in elderly individuals, particularly in VO2Max and cardiac perfusion. These enhancements are crucial for maintaining an independent lifestyle in older adults.
VO2Max, VO2Max/Kg, and VO2VT1 significantly increased in the HBOT group compared to the control group.
The HBOT group exhibited improved general cardiac perfusion, measured by Myocardial Blood Flow (MBF) and Myocardial Blood Volume (MBV).
The elderly rely heavily on VO2Max for daily tasks, making these improvements particularly relevant.
The study involved a total of 63 participants, divided into control and HBOT groups, with a focus on their health and demographic characteristics.
Average age of participants was 69.70 years, with 61.9% being male.
36.5% had a history of orthopedic surgery, and 47.6% had dyslipidemia.
Chronic conditions included hypertension (22%), diabetes mellitus (15.9%), and atrial fibrillation (6.3%).
Baseline measurements were taken to assess the initial health status of participants before the intervention.
Height, weight, BMI, and body surface area (BSA) were similar across groups, with no significant differences.
VO2Max was recorded at 1514.62 ± 521.17 mL/min, with no significant difference between groups.
VO2Max/Kg was 19.73 ± 5.93 mL/kg/min, also showing no significant differences.
The study evaluated changes in CPET parameters to assess the impact of HBOT on physical performance.
VO2Max increased significantly in the HBOT group (1517.98 ± 488.42 mL/min) compared to the control group (1584.70 ± 493.87 mL/min).
VO2VT1 showed a significant increase in the HBOT group (927.57 ± 308.28 mL/min) compared to baseline.
Power output and other secondary endpoints showed no significant changes.
The study highlights the effects of HBOT on cardiac perfusion, indicating potential benefits for heart health in elderly individuals.
MBF increased significantly in the HBOT group (0.30 ± 0.08) compared to baseline (0.34 ± 0.10).
MBV also showed significant improvement in the HBOT group (0.46 ± 0.12) compared to baseline (0.53 ± 0.14).
These changes suggest enhanced cardiac microcirculation due to HBOT.
The study's design allows for the isolation of HBOT effects, supporting the validity of the findings.
The findings suggest that HBOT can significantly enhance physical performance in aging adults, particularly in terms of oxygen consumption and cardiac function.
The study indicates that combining HBOT with physical training may yield synergistic effects, warranting further investigation.
The results underscore the potential of HBOT as a therapeutic intervention for improving quality of life in the elderly.

Previous studies have shown that hyperbaric oxygen therapy (HBOT) can improve the motor functions and memory of post-stroke patients in the chronic stage.
The aim of this study is to evaluate the effects of HBOT on overall cognitive functions of post-stroke patients in the chronic stage. The nature, type and location of the stroke were investigated as possible modifiers.
A retrospective analysis was conducted on patients who were treated with HBOT for chronic stroke (>3 months) between 2008-2018. Participants were treated in a multi-place hyperbaric chamber with the following protocols: 40 to 60 daily sessions, 5 days per week, each session included 90 min of 100% oxygen at 2 ATA with 5 min air brakes every 20 minutes. Clinically significant improvements (CSI) were defined as > 0.5 standard deviation (SD).
The study included 162 patients (75.3% males) with a mean age of 60.75 ± 12.91. Of them, 77(47.53%) had cortical strokes, 87(53.7%) strokes were located in the left hemisphere and 121 suffered ischemic strokes (74.6%). HBOT induced a significant increase in all the cognitive function domains (p < 0.05), with 86% of the stroke victims achieving CSI. There were no significant differences post-HBOT of cortical strokes compared to sub-cortical strokes (p > 0.05). Hemorrhagic strokes had a significantly higher improvement in information processing speed post-HBOT (p < 0.05). Left hemisphere strokes had a higher increase in the motor domain (p < 0.05). In all cognitive domains, the baseline cognitive function was a significant predictor of CSI (p < 0.05), while stroke type, location and side were not significant predictors.
HBOT induces significant improvements in all cognitive domains even in the late chronic stage. The selection of post-stroke patients for HBOT should be based on functional analysis and baseline cognitive scores rather than the stroke type, location or side of lesion.

This document discusses a 30-year experience with hyperbaric oxygen treatment for avascular necrosis of the femoral head and condyle.
Avascular necrosis (AVN) is a rare degenerative disease affecting 300,000–600,000 people annually. The femoral head is the most common site, accounting for 75% of cases. AVN can progress to joint destruction, often requiring surgical intervention. Risk factors include steroid use, diabetes, alcoholism, and decompression sickness. The Steinberg classification is used to grade AVN severity.
A retrospective chart review was conducted at Fremantle and Fiona Stanley Hospitals from 1989 to 2014. Patients treated with at least 20 sessions of hyperbaric oxygen therapy (HBOT) for AVN were included.
Changes in MRI results; secondary outcomes: subjective improvement and need for surgery.
21 joints in 14 patients were treated; 14 femoral heads and 7 femoral condyles. 64% of femoral head joints showed stable or improved MRI scans post-treatment. 71% reported good subjective outcomes; 3 joints required surgical intervention. In the femoral condyle group, all joints had stable or improved MRI scans, with no surgeries required.
AVN significantly impacts quality of life and is increasingly prevalent due to rising diabetes rates. HBOT may reduce edema and restore blood flow, potentially preventing disease progression. The study's results align with previous research, showing similar effectiveness of HBOT in treating AVN.
HBOT may prevent disease progression in femoral AVN, with results comparable to other studies. The study supports the role of HBOT in treating femoral AVN, showing both radiological and subjective improvements.

This document reports on the effectiveness of hyperbaric oxygen therapy for treating avascular necrosis of the femoral head.
The study involved 15 patients with Steinberg I-II avascular necrosis of the femoral head. Average follow-up was 22 months, with 13 patients showing satisfactory outcomes. Pain scores improved significantly (P<0.001), and 26.7% of hips progressed to collapse. No complications were reported, indicating safety and effectiveness of hyperbaric oxygen therapy.
Avascular necrosis (AVN) can lead to debilitating conditions and major surgeries. Early intervention is crucial to prevent joint arthroplasty.
Hyperbaric oxygen (HBO) therapy is a less invasive alternative that improves microcirculation and reduces bone edema.
Data collected from a registry of patients treated between January 2010 and December 2018.
Adults with non-traumatic Steinberg stage I-II AVN confirmed by MRI.
Patients received 25-40 sessions of HBO therapy, with clinical evaluations conducted at regular intervals.
15 patients (17 hips) treated; mean age was 36 years, with 47% females.
73.3% were Steinberg stage II; 86.7% had satisfactory outcomes with an average Oxford hip score of 37.3. Pain scores decreased from 5.1 to 1.5 (P<0.001), with no complications reported.
HBO therapy showed a high rate of satisfactory outcomes (P=0.001).
26.7% of cases progressed to collapse, but all had satisfactory outcomes at follow-up. More high-level studies are needed to validate HBO's effectiveness in AVN treatment.
Previous studies reported significant improvements in pain scores and hip range of motion with HBO therapy. Comparisons with untreated groups showed HBO significantly reduced the likelihood of lesion progression.
Studies indicated that HBO therapy can lead to stable disease and radiographic improvement over time. Higher effectiveness noted in earlier stages of AVN.
HBO therapy influences the OPG/RANKL system, which is crucial in bone metabolism. Significant increases in soluble OPG were observed after HBO treatment.
HBO is a safe and effective treatment for pre-collapse AVN of the femoral head, with low rates of progression to collapse. Further validation through larger-scale trials is necessary.

This document reviews the role of hyperbaric oxygen therapy (HBOT) in treating idiopathic sudden sensorineural hearing loss (ISSNHL), summarizing its clinical effects and proposed mechanisms.














月曜日-金曜日 | 午前 9 時-午後 7 時
土曜日 | 午前9時~午後3時
日本、〒104-0061 東京都中央区銀座5丁目−10−12 東洋スビル 3F

